Older patients (pts) with newly diagnosed ALL are ineligible for unmodified pediatric-based therapies. Although results are improved with age-adapted, dose-reduced regimens including MRD-based treatment modification, there is much space for further improvement.Therefore, the GMALL study group developed a trial to evaluate Blinatumomab, a CD19 directed bi-specific T-cell engager, in sequence with chemotherapy in this patient population with high medical need (NCT 03480438). Importantly, Blinatumomab replaced three cycles of standard consolidation chemotherapy.Pts aged 56 - 76 years (yrs) with CD19-positive, Ph-neg B-precursor ALL wereeligible. The primary endpoint was complete hematologic remission (CR) afterinduction (1 cycle chemo, 1 cycle Blinatumomab) and the key secondary endpoint was molecular response (table 1). The efficacy population included pts treated with dose reduced and shortened induction I (IP1) and Blinatumomab 1 or early death (ED). Induction was followed by 3 further Blinatumomab cycles alternating with age adapted consolidation cycles (HDMTX/ASP, HDAC, reinduction and maintenance) according to GMALL protocols for older pts (Gökbuget et al, ASH 2021).
The median age was 66 (56-76) yrs. 21% had pro-B-ALL and 79% c/preB subtype. 40% had comorbidities according to the Charlson Score; most frequent were diabetes (13%) myocardial infarction (10%) or vascular diseases (10%).
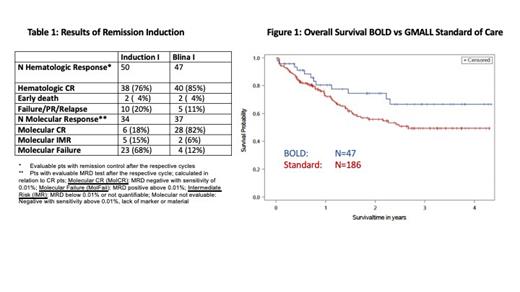
50 pts were evaluable for IP1: 76% achieved CR/CRu and 2 pts (4%) died due to infections. 33% (N=11) had a molecular response and 18% MolCR (table 1). 47 pts were evaluable for the primary endpoint after Blinatumomab 1: CR/CRu was observed in 85%; 2 experienced ED (4%; those mentioned above; no additional ED). 88% of the CR pts had a molecular response (82% MolCR).
The median follow-up is 757 (61-1584) days. Overall Survival (OS) after 1 and 3 yrs was 80% and 67% for the efficacy population (figure 1). The resp results for the total population were 82% and 65%. The 3y OS was 69% for c/pre-B-ALL and 58% for pro-B-ALL (p>.05). 3y OS was 81% for pts aged 55-65 yrs and 53% for those older than 65 yrs (p=.025). Only three pts received an allogeneic stem cell transplantation in CR1. 6 pts relapsed, 2 pt developed a secondary malignancy (Colon Ca, MDS) and 3 pts died in CR (1 HLH, 1 arterial disease, 1 Covid19). EFS was 60% at 3 yrs.
We compared the results with those of the current GMALL standard therapy for older pts with B-precursor ALL (N=186) (Gökbuget et al, ASH 2022). Patient characteristics were similar. CR rates after induction were 85% vs 78% for BOLD vs standard (p>.05). Molecular CR rates in pts with hematologic CR were 82% vs 55% (p=.006). OS at 3 yrs was 67% and 49% (p=0.08) and Remission Duration (RD) was 83% vs 58% (p=0.055).
Overall tolerability and efficacy of the regimen was very promising with a high hematologic and molecular response rate; mortality in induction and consolidation was low. In comparison with the current standard therapy the MRD response rates were significantly better. OS and RD was superior despite the omission of several chemotherapy cycles in contrast to other trials with unmodified chemotherapy but additional cycles of Blinatumomab. An ongoing randomized trial (NCT04994717) compares an even more dose reduced combination of Blina and chemotherapy with standard of care.
This is an independent academic trial initiated by the GMALL Study Group which received free supply of Blinatumomab and financial support by Amgen.
OffLabel Disclosure:
Goekbuget:Servier: Honoraria, Research Funding; Clinigen: Honoraria, Research Funding; Autolus: Honoraria; Incyte: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Research Funding; Gilead: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Schwartz:Pfizer: Consultancy, Honoraria; Amgen: Other: Advosory Board; Protherics: Research Funding. Topp:GenMab: Consultancy; Takeda: Research Funding; Regeneron Pharmaceuticals, Inc.: Consultancy, Research Funding; AbbVie: Consultancy; Kite, a Gilead Company: Consultancy, Other: Travel support, Research Funding; Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy, Other: Travel support; Bristol Myers Squibb: Consultancy, Research Funding. Subklewe:Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Seagen: Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead/Kite: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding; Ichnos Sciences: Consultancy, Honoraria; AstraZeneca: Speakers Bureau; Pfizer: Consultancy, Honoraria, Other: Travel Support, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AvenCell: Consultancy, Honoraria; Incyte Biosciences: Consultancy, Honoraria; Molecular Partners: Consultancy, Honoraria, Research Funding; GSK: Speakers Bureau; LAWG: Speakers Bureau; Springer Healthcare: Speakers Bureau; AbbVie: Consultancy, Honoraria; Autolus: Consultancy, Honoraria; advesya (CanCell Therapeutics): Consultancy, Honoraria; Genmab US: Consultancy, Honoraria; Interius BioTherapeutics: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Orbital Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Scare: Consultancy, Honoraria. Vucinic:Sobi: Honoraria, Other: Travel/Accommodations/Expenses; Abbvie: Honoraria; Janssen: Honoraria; MSD: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AstraZeneca: Honoraria; BMS/Celgene: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Gilead/Kite: Consultancy, Honoraria; Amgen: Honoraria; Novartis: Consultancy, Honoraria. Brüggemann:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Regeneron: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; BD: Speakers Bureau; Janssen: Speakers Bureau; Pfizer: Speakers Bureau; Affimed: Research Funding. Viardot:F. Hoffmann-La Roche Ltd, Abbvie, Kite/Gilead, BMS: Honoraria; F. Hoffmann-La Roche Ltd, Abbvie, Kite/Gilead, BMS: Consultancy; BMS: Research Funding.
Blinatumomab in first line

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal